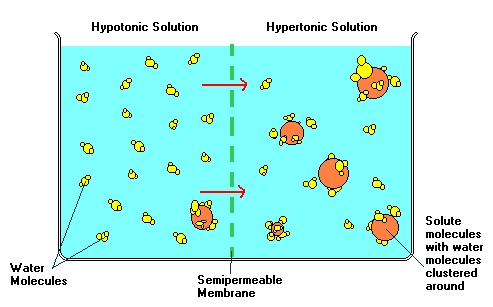
In the diagram above, a solute has just been added to the right of
the membrane. This addition does not change the number of water molecules
that are in the container, but it does impose new restrictions on the movement
of the water molecules. The water molecules that have clustered around
the new solute are not able to freely travel through the membrane, because
they are attatched to the solute molecules. But the single water
molecules are still able to travel either way across the membrane.
The water will move from the left (hypotonic) side to the right (hypertonic)
side, because the right side has a lower concentration of free water molecules.
The force that makes the molecules move like this is called the osmotic
pressure.
Home